Detail
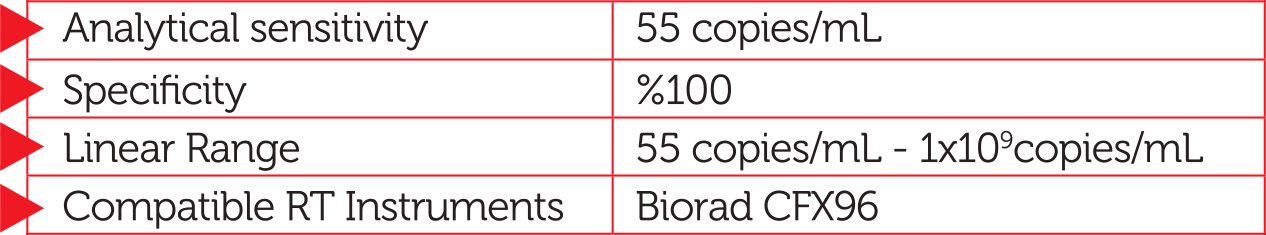
RTA VZV Real—Time PCR Kit is an in vitro nucleic acid amplification assay for the quantitation of Varicella Zoster Virus DNA in human serum samples. The kit is designed to be used as an aid in the management of cure for transplantation patients with potential VZV infection in conjunction with all relevant clinical and laboratory findings.
RTA VZV Real—Time PCR Kit provides you everything needed for a reliable and efficient detection:
- Very high sensitivity of detection
- Extended linear range
- Complete specificity
- Whole process check by adding the internal control during extraction
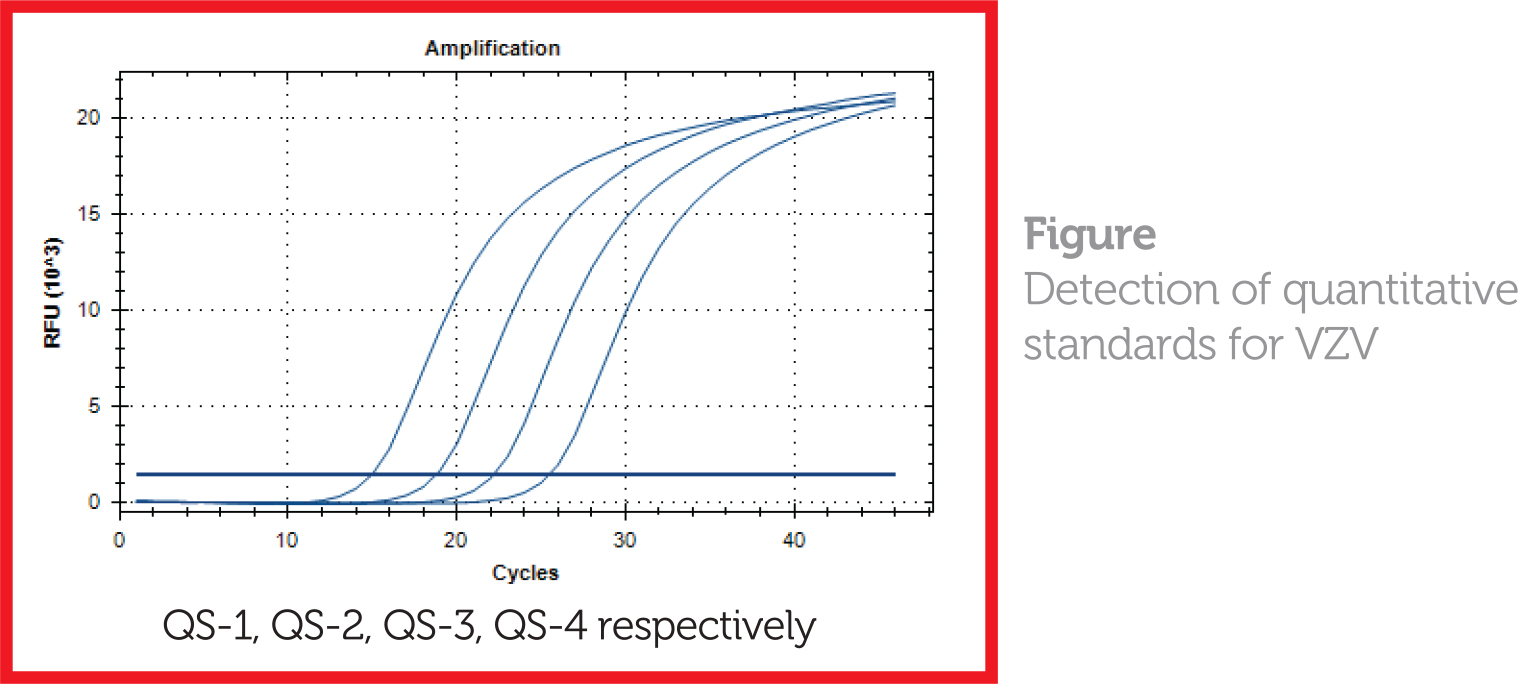
- 4 external quantitation standards calibrated against the WHO CE
- marked reference materials
- Compatibility with widely used thermal cyclers
- 98/79/EC EU IVD Directive compliance
RTA EBV Real-Time PCR Kit is perfectly designed to amplify and detect a highly conserved region within the EBV genome by Real Time PCR. It is possible to monitor the nucleic acid amount on-line, during the reaction utilizing hydrolysis probe method in which fluorescence emission increases proportionally to the DNA amount.
Sample Preparation: Nucleic acid extraction is a decisive step prior to qPCR since; providing high yields of nucleic acid and removal of PCR inhibitors in turn improve the amplification efficiency and critical for avoiding false negative PCR results.
RTA VZV Real-Time PCR Kit has been validated for use with RTA Viral DNA Isolation Kit for its best performance.